electron configuration of fe|How do you determine the electron configuration of Fe? : Bacolod Potassium (K) - Electron Configuration for Iron (Fe, Fe2+, and Fe3+) - UMD Season 1. Season 1; Season 2; Season 3; Set in 43 A.D., Britannia is an epic and visionary drama that follows the Roman invasion of Britain when the country was ruled by the powerful Druids and warrior queens. . The Celts of 43AD Britannia are a divided and warring group of tribes. King Pellenor of the Cantii has to manage his strong willed .
PH0 · Iron Electron Configuration (Fe) with Orbital Diagram
PH1 · Iron
PH2 · How do you determine the electron configuration of Fe?
PH3 · How do you determine the electron configuration of Fe?
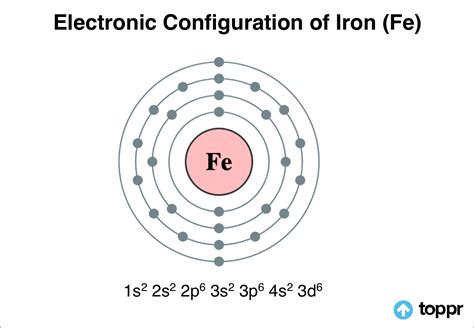
PH4 · Electronic Configuration of Iron: Fe element
PH5 · Electronic Configuration of Iron
PH6 · Electron Configuration for Iron (Fe, Fe2+, and Fe3+)
PH7 · Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions)
PH8 · Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions)
PH9 · Electron Configuration Chart of All Elements (Full Chart)
PH10 · 6.4 Electronic Structure of Atoms (Electron Configurations)
Sometimes, no deposit is required for a free spins bonus, depending on the deal. We specify how to claim every free spins bonus in the table of offers above. 5 . Use Your Free Spins . After following the steps above, you can enjoy your free spins bonus. Just follow the playthrough requirements to give yourself the best chance of converting .
electron configuration of fe*******Learn how to write the electron configuration for iron and its ions using the period table or an electron configuration chart. See the video, examples and step-by-step tutorial for writing the electron configurations.
In order to write the Calcium electron configuration we first need to know the .
Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s .How to Write the Electron Configuration for Nitrogen (N) Nitrogen is the seventh .
Potassium (K) - Electron Configuration for Iron (Fe, Fe2+, and Fe3+) - UMDIn order to write the Mg electron configuration we first need to know the .Chlorine (Cl) - Electron Configuration for Iron (Fe, Fe2+, and Fe3+) - UMDIn order to write the Sulfur electron configuration we first need to know the .
Iron (Fe, Fe 2+, Fe 3+) Read my . Electron Configuration Notation:-shows the .119 rows — Mar 23, 2023 — Find the electron configuration of iron (Fe) and other .

Learn the electronic configuration of iron (Fe) with 26 electrons, its oxidation states, and its applications. Find out how iron forms different allotropic forms .Learn how to write the electronic configuration of iron (Fe) and its oxidation states (+2 and +3) with examples and diagrams. Also, find out the valency and applications of iron in various fields.Hul 29, 2021 — Electron Configuration for Fe. The iron electron configuration is one of the other significant areas of study for scholars of chemistry. The electron configuration for any chemical .Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. Boiling pointThe .Mar 18, 2018 — Learn how to determine the ground state electron configuration of iron (Fe) using the Aufbau diagram and the periodic table. See the answer and explanation from two different sources.Ago 21, 2024 — The electronic configuration of \[Fe^{2+} is - 1s^{2} 2s^{2} 2p^{6} 3s^{2} 3p^{6} 3d^{6},\] while that of \[Fe^{3+} is 1s^{2} 2s^{2} 2p^{6} 3s^{2} 3p^{6} 3d^{5}\]. \[Fe^{2+}\] .
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of .The first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers and electron .This page explains the theory behind writing electron configurations of atoms. Skip to main content +- +- chrome_reader_mode Enter Reader Mode . (II) loses two electrons and, since it is a transition metal, they are removed from the 4s .
Hun 28, 2019 — In order to write the electron configuration for Iron (Fe) we first need to know the number of electrons for the Fe atom (there are 26 electrons).Nob 26, 2023 — Start with the electron configuration of the neutral atom. It is sufficient to use the noble gas configuration. Fe: [Ar] 4s 2 3d 6. Then, remove (or add) electrons to reflect the oxidation state of the metal. Remember to remove electrons from the orbital with the highest principal quantum number first! Fe(III) has lost 3 electrons relative to .Hun 30, 2023 — Example of Determining Energy Levels (n) For example, if we want to determine the electron configuration for Cobalt (Co) at ground state, we would first look at the row number, which is 4 according to the periodic table below; meaning n = 4 for the s-orbital.In addition, since we know that the energy level for the d orbital is "n-1", therefore n = 3 for the d-orbital in this case.When compared to the electronic configuration of Fe, Fe 2+ has two fewer electrons while Fe 3+ has three fewer electrons. Valency of Iron. Valence electrons are a single outer shell electron in an atom that is responsible for the chemical properties of the atom. An element’s valency is the number of electrons it receives, gives away, or .Hun 20, 2023 — The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and 14. Thus in the .The valence electrons (here 3s 2 3p 3) are written explicitly for all atoms. Electron configurations of elements beyond hassium (element 108) have never been measured; predictions are used below. As an approximate rule, electron configurations are given by the Aufbau principle and the Madelung rule.Ene 30, 2023 — To write the electronic structure for Fe 3 +: Fe: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2; Fe 3 +: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5; The 4s electrons are lost first followed by one of the 3d electrons. This last bit about the formation of the ions is clearly unsatisfactory. . The Full Story of the Electron Configurations of the Transition Elements .
Electron configuration 3d 6 4s 2: Electrons per shell: 2, 8, 14, 2: Physical properties; . resulting in a Fe–O–O bond angle of around 120° that avoids the formation of Fe–O–Fe or Fe–O 2 –Fe bridges that would lead to electron transfer, the oxidation of Fe 2+ to Fe 3+, and the destruction of hemoglobin. This results in a movement .
How do you determine the electron configuration of Fe? Hul 29, 2021 — Electron Configuration for Fe. The iron electron configuration is one of the other significant areas of study for scholars of chemistry. The electron configuration for any chemical element is basically the process that defines the electrons distribution process of the element to its atomic orbitals.Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. . What is the electronic configuration of #Fe^(3+)# ion? Question .Nob 13, 2020 — Iron is a chemical element with atomic number 26 which means there are 26 protons and 26 electrons in the atomic structure.The chemical symbol for Iron is Fe. Electron Configuration and Oxidation States of Iron. Electron configuration of Iron is [Ar] 3d6 4s2. Possible oxidation states are +2,3. Electron Configuration. The periodic table is a tabular .electron configuration of feFe–N 4 sites anchored on carbon (Fe–N–C) materials have drawn increasing interest on account of their remarkable electrocatalytic activity for the oxygen reduction reaction (ORR). Nevertheless, the Fe–N 4 sites with a symmetric electronic configuration exhibit too strong binding energy between the Fe center and oxygen intermediates toward the ORR.
The electron configuration of Fe (1s2 2s2 2p6 3s2 3p6 4s2 3d6) tells us that there are a total of 26 electrons. In the orbital diagram, we represent each orbital by a box and use arrows to represent the electrons. The boxes are organized according to the different energy levels, with the lowest energy level at the bottom and the highest energy .Ene 13, 2023 — The electron configurations and orbital diagrams of these four elements are: Figure \(\PageIndex{5}\): Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches the core electron configuration, along with the valence electrons in a condensed .Step 1: Find the electron configuration. For Cl atoms, the electron configuration is 3s 2 3p 5. Step 2: Draw the valence orbitals . The Fe 2 + ion has 3d 6 has the electron configuration. Because it has 4 unpaired electrons, it is paramagnetic. Contributors . Modified by Ronia Kattoum (UA of Little Rock)electron configuration of fe How do you determine the electron configuration of Fe? Ago 21, 2024 — \[Fe^{2+}\] contains 2 fewer electrons compared to the electronic configuration of Fe. In order to write the Iron Electron Configuration, we need to know the number of electrons for the GFe atom, and once we have the configuration for Fe, the ions are simple. When we write the electronic configuration the first two electrons go to the 1s orbital.
Conoce el perfil de Rudy Gobert, Centro de Minnesota Timberwolves en ESPN DEPORTES. Entérate de las últimas noticias, estadísticas en vivo y mira los highlights.
electron configuration of fe|How do you determine the electron configuration of Fe?